Translin/trax: a microRNA-degrading enzyme
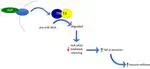
Expression of genes can be regulated at several levels in different ways. The microRNA system is one way of controlling which genes will be expressed i.e. form proteins. MicroRNAs are ~ 22 nucleotide long segments of RNAs. A cell has hundreds of microRNAs. Each microRNA can bind to dozens of messenger RNAs (mRNAs) and prevent these mRNAs from forming proteins, hence silencing them. If the cell needs to synthesize these proteins in response to extracellular signals, it needs a way to degrade the microRNA and reverse gene silencing (release the brake). There are several enzymes that degrade subsets of microRNAs. These could help regulate gene expression in a precise manner. We are investigating the role of one such microRNA-degrading enzyme called the translin/trax (TN/TX) complex in health and disease. One way of addressing this research question is to study the impact of loss of the TN/TX complex. Accordingly, we are studying the traits that are presented by TN KO mice that lack the TN/TX complex (due to deletion of the translin gene from their genome).We observed that these mice have a robust increase in fat mass. Excess fat is a major risk factor for metabolic diseases such as diabetes. Yet, surprisingly, TN KO mice do not have any impairment in glucose tolerance. We characterized the mice further to determine what was contributing to this ‘protection’ and identified three potential factors. Unlike, mice who have a comparable increase in fat (due to a high-fat diet), TN KO mice have 1) elevated levels of adiponectin, a protective agent released by fat cells, 2) decreased inflammation and 3) a disproportionate increase in subcutaneous fat instead of visceral fat. The former is less harmful than the latter. It is likely that collectively, these factors may be attributing protection from the detrimental consequences of increased fat in these mice. These findings have implications for treating metabolic diseases such as diabetes.
Neuronal plasticity is essentially brought about by changes in number or strength of synapses (connections) between neurons. This phenomenon underlies several brain diseases and can bring about changes in behavior. MicroRNAs may be critical drivers of this phenomenon. Accordingly, we are exposing mice to different stimuli that are known to induce changes in neuronal plasticity and assessing if TN/TX protein level or enzyme activity is increased. Currently, I am investigating if long-term stress exposure can alter the TN/TX levels or activity in a brain region known to have changes in plasticity in response to stress. If this happens to be the case, I will use genetically-modified mice that lack TN/TX specifically in this brain region and check if these mice are more prone to the detrimental effects of stress. We are also testing behavioral and neurochemical responses to various drugs of abuse in genetically-modified mice that lack the TN/TX enzyme in neurons that produce and release dopamine, the primary neurotransmitter involved in addiction. These studies will help us understand the role of this enzyme in diseases such as depression and substance abuse.